Untitled Document
© 2014 Philipp Fürnstahl; Lizenznehmer RTejournal, weitere Informationen sind zu finden unter: http://www.dipp.nrw.de/service/dppl/
urn:nbn:de:0009-2-39466
The computer assisted research and development (CARD) group was founded by the University Hospital Balgrist to establish a new research focus in patient-specific orthopedic surgery. The CARD group pursues not only basic research but also performs application-driven development, promoting the incorporation of research findings into clinical practice. In 2009 the University Hospital Balgrist reported on early results on the computer assisted 3D planning of two complex radius shaft malunions [1, 2]. The developed surgical planning environment, which is now part of the CARD planning services, allowed the exact 3D quantification of bone malunion in all 6 degrees of freedom (i.e., translation and rotation) and the simulation of the corrective osteotomy by matching the 3D shape of the affected radius model with the mirrored model of the opposite side.
So far, over 100 patients were successfully treated by corrective surgeries of the upper [3] and lower limbs [4] at our institution using 3D preoperative planning combined with rapid-prototyped surgical guides. In this article we describe our experience in this field by means of example cases. An overview of our work including additional surgical example cases can be found on our homepage http://card.balgrist.ch. We offer solutions and services for orthopedic surgeries using dedicated software products and patient-specific instrumentation.
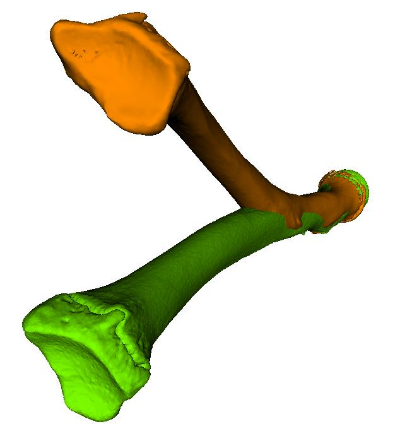
Figure 1: Severe bone deformity (orange) compared to the opposite healthy bone (green).
A prerequisite for designing patient-specific surgical guides is that a virtual plan of the surgery is generated based on 3D bone models of the patient anatomy, extracted from computed tomography (CT) images [5] (i.e., segmentation). In malunited bones, which heal in non-anatomical position after fracture, the bone deformity can be quantified by using a mirror-model of the opposite healthy bone as a 3D reconstruction template (see Figure 1). Several techniques can be applied to perform the alignment task in an automatic fashion. Commonly used are surface registrations algorithms, such as iterative closest point registration [6], allowing to calculate the optimal alignment with respect to the model points in a least-square sense. Nevertheless, the alignment task must be performed with caution, since such registration methods are sensitive to the initialization (i.e., the initial configuration between the models to be aligned) and outliers [7]. Another issue related to 3D planning with a reconstruction template is that the influence of soft tissue structures, such as ligaments, are completely ignored. As a consequence, a surgical correction with respect to the opposite anatomy is not always optimal, as demonstrated in Figure 2. In this patient, the 3D bone comparison revealed no clinically significant deformity although the range of motion of the forearm rotation (i.e., pro-supination) was impaired by more the 40º.
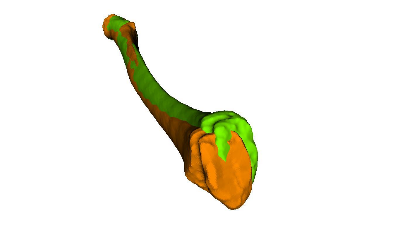
Figure 2: In this case a serious functional impairment was present, although the bone malunion (orange) was clinically not relevant compared to the opposite healthy bone (green).
The surgical guides must be sterilized after additive manufacturing. Sterilization may be performed directly by the manufacturer using irradiation with gamma rays or, otherwise, by the hospital. Most hospitals including our institution perform sterilization using conventional steam pressure, since gamma radiation based sterilization is typically not available. We have conducted an experiment to study the effect of steam pressure sterilization on material deformity, i.e., we measured if and how the plastic guides deform under high temperature (i.e., 130º for 15 to 20 minutes). Two different systems and biocompatible materials were included in the test, namely selective laser sintering (SLS) with polyamide PA 2200 as raw material (EOS GmbH, Krailling, Germany) and multi-jet printing with VisiJet M3 Crystal as raw material (3D systems, Rock Hill, SC, USA).
A test guide model was designed, manufactured, and 3D-scanned before and after sterilization to allow comparison with the original model. As expected, the guides before sterilization matched the ground-truth with high accuracy (i.e., within 0.2 mm) regardless of the manufacturing technology or material used. The guide bodies deformed slightly after sterilization as shown in Figure 3. The highest error of 0.5 mm was observed for thin-walled drilling sleeves having a very small wall thickness (i.e., 0.5 mm). Therefore, we recommend using at least structures with a minimum wall thickness of 1 mm if steam pressure sterilization is applied.
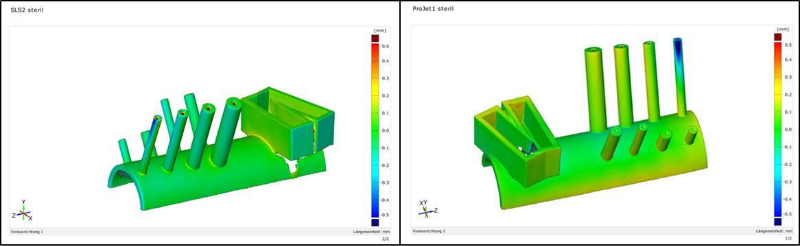
Figure 3: Deformity of the guide shape after steam pressure sterilization for SLS-manufactured PA 2200 material (left) and jet-printed VisiJet Chrystal material (right).
Different methods were described to reproduce a 3D surgical plan during operation. Patient-specific guides are often preferred by surgeons since the application is easier compared to complex navigation systems. The guides are molded to conform the bone surface, allowing to uniquely identify the location on the bone were it has to positioned during surgery. The accuracy of the surgery is therefore also dependent on how well the guide fits to the bone surface. A tight fit can be easier achieved in irregularly shaped bone regions (e.g., closer to the joints) in contrast to mid-shaft regions due to the lack of unique landmarks on the cylindrically shaped shaft surface. The translation along and the rotation around the bone axis are therefore the least constrained degrees of freedom during positioning in the mid-shaft region.
Cutting guides are used to cut (i.e., osteotomize) the bone exactly as virtually defined. Reduction guides support the surgeon in the task of reducing the bone fragments according 3D surgical plan. As depicted in Figure 4, a rapid-prototyped cutting guide permits to define the position and orientation of the blade during sawing. A metallic device is inserted into the dedicated slot of the guide body before cutting to avoid direct contact between the saw blade and the polyamide guide, yielding a more precise and debris-free cut. The CE-certified metallic block, which has been developed by Balgrist CARD, can be reused. A quantitative evaluation of the cutting accuracy was carried out in one case in which a closing wedge osteotomy was performed (i.e., a wedge was removed by making two cuts). As depicted in Figure 4 b to c, a CT of the removed wedge was acquired and the extracted 3D model was compared to the planned wedge, showing an error of 0.4 mm which is within the thickness of the saw blade.
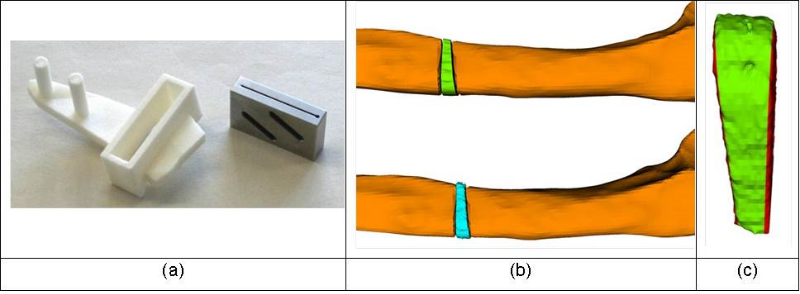
Figure 4: (a) The patient-specific cutting guide (left) is used together with the manufactured cutting block (right). (b) Comparison of a closed-wedge osteotmy between planned resection (top) and surgery (bottom). (c) Cutting accuracy: Difference between planned and performed wedge resection is denoted in red.
Fragment alignment after cutting is either achieved with a separate reduction guide based on Kirschner wires (K-wires) or by pre-drilling screw holes of the fixation plate. An example of using the later method in a distal tibia osteotomy is given in Figure 5. Pre-drilling the screw holes according to the screws in their postoperative position has the benefit that no separate reduction guide is needed and, thus, a smaller incision is required. Although guide positioning is accurate in general (see Figure 5 b), we have experienced that inaccuracies can arise if a screw locks into the plate differently as planned preoperatively (see Figure 5 c). It may be additionally difficult to precisely maintain the planned position of the fragment without separate reduction guide in the presence of high soft tissue tension. Correction of the axial/rotational component is particularly sensitive to these issues, which can cause residual error of up to 8º.
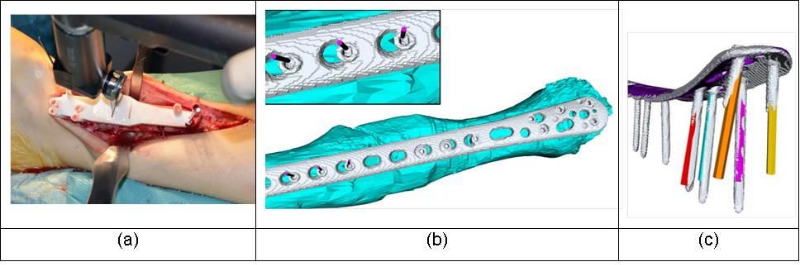
Figure 5: (a) Patient-specific guide with predrilled plate holes. (b) Accuracy of guide positioning: planned screw center (magenta cylinders) compared to the final screw placement in the surgery (grey). (c) Screw directions of the preoperative planning (colored) versus screw direction of the surgery (grey).
For this reasons we recommend using a separate reduction guide as described in our previous work [8]. By doing so a very accurate procedure is possible (i.e., residual error below 2º and 2 mm on average).
We have further developed our technique to permit application to smaller anatomy such as bones of the wrist and hand. So far we have successfully applied 3D preoperative planning and/or rapid-prototyped surgical guides in the treatment of over 20 malunited scaphoid bones (i.e., 5 cases with guides) and 10 malunited bones of the hand (i.e., 6 cases with guides). A corrective osteotomy of a malunited scaphoid bone using a reduction guide is shown in Figure 6. Although correct placement of the small guides on the bone surface may be less accurate compared to the forearm, our early experiences indicate a more controlled procedure compared to the conventional approach.
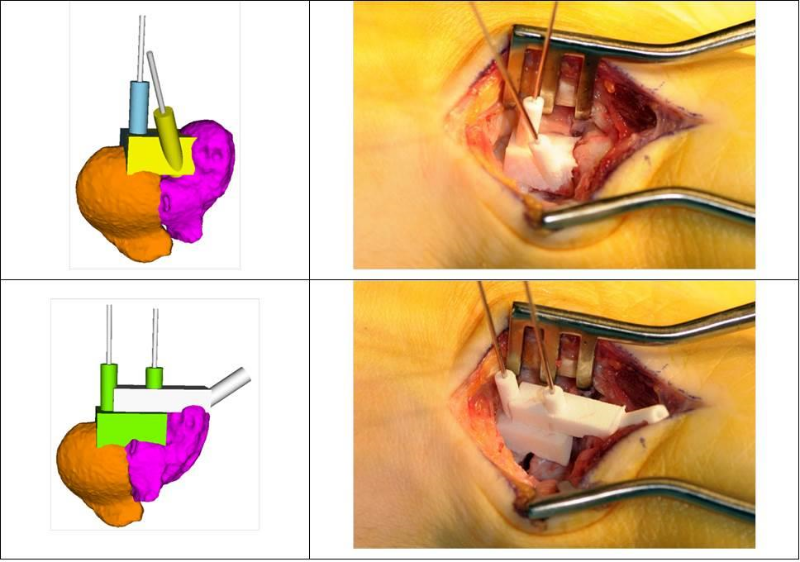
Figure 6: Corrective osteotomy of a malunited scaphoid bone using a reduction guide before (top row) and after reduction (bottom row).
1. Fürnstahl P, Fuchs T, Schweizer A, Nagy L, Szekely G, Harders M. Computer-aided osteotomy planning. Proc CAOS International 2009; 2009; Boston, USA.
2. Schweizer A, Fürnstahl P, Harders M, Szekely G, Nagy L. Complex radius shaft malunion: osteotomy with computer-assisted planning. Hand (N Y). 2010 Jun;5(2):171-8.
3. Nagy L, Fürnstahl P, Schweizer A. Computerassistierte 3-D-Technologie in der orthopädischen Handchirurgie. Leading Opinions Orthopädie und Rheumatologie. 2014 Jul(4).
4. Fürnstahl P. Computer Assisted Orthopedic Surgery Planning with Rapid Prototyping. Proc of the 10th RapidTech Conference; 2013; Erfurt, Germany.
5. Fürnstahl P, Fuchs T, Schweizer A, Nagy L, Szekely G, Harders M. Automatic and robust forearm segmentation using graph cuts. Biomedical Imaging: From Nano to Macro, 2008 ISBI 2008 5th IEEE International Symposium on; 2008 14-17 May 2008.
6. Besl PJ, McKay ND. Method for registration of 3-D shapes. Robotics-DL tentative; 1992: International Society for Optics and Photonics.
7. Rusinkiewicz S, Levoy M. Efficient variants of the ICP algorithm. 3-D Digital Imaging and Modeling, 2001 Proceedings Third International Conference on; 2001: IEEE. ISBN: 0769509843.
8. Schweizer A. New technologies in planning and performance of osteotonics: example cases in hand surgery. Praxis (Bern 1994). 2013 May 8;102(10):579-84.
Dr. Sc. Philipp Fürnstahl
Computer Assisted Research & Development Group, Universitätsspital Balgrist, Universität Zürich
Forchstrasse 340, 8008 Zürich, Schweiz
philipp.fuernstahl@balgrist.ch
,
card.balgrist.ch
Dr. med. Stephan Wirth
Orthopädie, Universitätsspital Balgrist, Universität Zürich
Forchstrasse 340, 8008 Zürich, Schweiz
stephan.wirth@balgrist.ch
,
www.balgrist.ch
Prof. Dr. med. Ladislav Nagy
Orthopädie, Universitätsspital Balgrist, Universität Zürich
Forchstrasse 340, 8008 Zürich, Schweiz
ladislav.nagy@balgrist.ch
,
www.balgrist.ch
PD Dr. med. Andreas Schweizer
Orthopädie, Universitätsspital Balgrist, Universität Zürich
Forchstrasse 340, 8008 Zürich, Schweiz
andreas.schweizer@balgrist.ch
,
www.balgrist.ch