Bones consists of up to 60-70% of inorganic com-pounds, whereof the biggest part is hydroxyapatite (HA). Today synthetic HA material is widely used as bone substitutes. The biological acceptance of such material, which is similar to human bone, is much higher compared to common metal alloys for implants. HA material is absorbable and can be replaced by the body’s own bone material. This is why HA based bone substitutes have big advantages in bone healing with-out removing such an implant after bone regeneration. [2]
If a HA sample printed with an acid binder is sintered, a partially degradation of HA to tricalcium phosphate with a higher degradation rate and calcium phosphate takes place. Specific and controlled degradation of HA allows to adjust the dissolving procedure and optimiz-ing the ingrowth process. After implantation of a HA implant the body interacts with the implant and a bone dissolving and bone growing process starts. To support this process in the body, human biology and material chemistry have to be known well. To avoid thermal degradation non-acid binders were investigated.
In terms of the extraordinary compressive strength and simultaneous bending properties of the bone, the me-chanical properties of the implants should be as simi-lar as possible. Therefore composite structures with gelatine were produced and CS was tested.
By the 3D printing process a roller spreads out the used powder for each layer whereby the structure is built up layer by layer. After spreading out binders are used to stick powder particles in each layer together. 3D printing allows to build up freeform structures which could not be manufactured with conventional manufacturing processes. One important aspect during the 3D printing process is that these powder particles could be deposited as close as possible. The closer these powder particles could be deposited the better the particles can be sintered afterwards. The aim is to have always an equal and commensurate sintering activity. Not every manufacturing process leads to an evenly dense microstructure with a high sintering ac-tivity like high pressure compressed powder samples. To test if the sintering activity of 3D printed samples could be increased, several additives were examined in a preliminary phase.
Materials
Dextran (from Leuconostoc spp.) was obtained from Sigma (31390-25G) and hydroxyapatite from Medi-coat AG (Mägenwil, Switzerland). Polyvinyl alcohol, poly--caprolactone (PCL) and polyethylene glycol (PEG) were used for binder tests. D(+)-sucrose (Carl Roth) was used as binder during the printing process. To study the sinter activity of different additives po-tassium carbonate (K2CO3), sodium carbonate (Na2CO3), potassium fluoride (KF) and sodium tri-phosphate (Na5P3O10) were used. Powdered gelatine (Merck) was used for the infiltration.
Production of specimens
A modified 3D printing system (Z-Corp, Z-510) was used to produce HA and HA/polymer full body cylin-ders (Ø 10 x 10mm) as well as cubic samples with an open porous structure (10 x 10 x 10mm). Two ap-proaches were used to print HA/polymer samples. On one hand pure HA (Medicoat, Medipure) was used as bulk material and polymers were only used as binders. On the other hand HA was blended with 15 wt.% dex-tran as bulk material and printed with polysaccharide based binders.
Samples for the infiltration were printed with an acid based binder. Full body cylinders for the binder testing were printed with various polysaccharide binders. All thermal treatments were executed with the furnace RHF 15/3 from Carbolite.
Infiltration of 3D printed samples
The infiltration was performed with printed and sin-tered samples with a defined open porous structure. The samples were infiltrated by means of different methods with gelatine under several concentrations of 10 wt.%, 12 wt.% and 20 wt.% at 50°C.
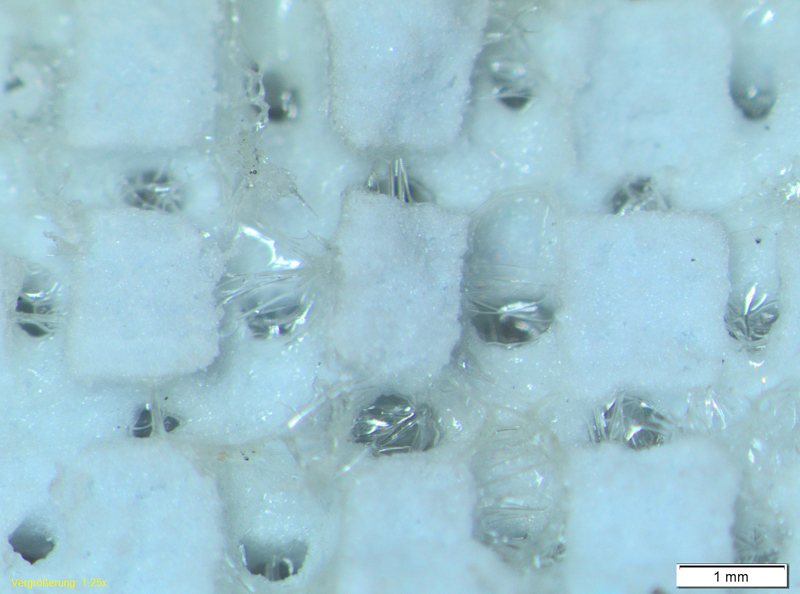
Fig. 1: Inside of a dried and sliced 3D printed sample after infiltration with 10 wt.% gelatine with method 1 for four times.
In method 1 the sample was fixed in an infiltration cell (Fig. 2) whereby the medium was pumped through the sample. The whole process took place under a defined vacuum. After one run the sample was dried for at least 12h. In method 2 the sample was dipped into the infil-tration solution, a defined vacuum was set for a certain time and afterwards dried for at least 24 hours.
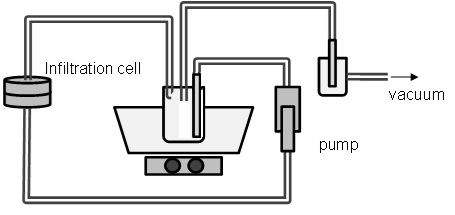
Fig. 2: Infiltration model for method 1. The image shows the infiltration cell (sample holder) where the medium is pumped through the sample. The whole apparatus is under a defined vacuum.
Increasing of the sintering activity
Based on studies [3] and [4] where sintering additives were investigated to increase sintering activity for pressed HA test specimens, the effect of additives for non-pressed samples would be determined. For the preliminary study pure HA was blended with different additives like potassium carbonate (K2CO3), sodium carbonate (Na2CO3), potassium fluoride (KF) or sodi-um triphosphate (Na5P3O10). Respectively one additive was dissolved in ultra-pure water (up. water) mixed with pure HA with a ratio HA/additive of 5 wt.% and stirred with a spatula to a homogeneous suspension. The suspension was casted into a mould (cylinders Ø 10 x 10 mm), dried for 2 hours and sintered afterwards.
Analysis
The mechanical properties and the surface characteri-sation were investigated and performed by studies of compressive strength (Hydropulser LFV-5-PA/ECD 120, walter+bay ag), Vickers hardness, porosity by Archimedes and scanning electron microscopy (SEM TM-1000 Tabletop, Hitachi). To verify the degradation of HA during the sintering process X-ray diffraction measurements (XRD, D2 Phaser, Bruker) were per-formed. For the XRD measurements an X-ray power of 30kV, soller modules left and right of 2.5°, a variable rotation of 10 rpm and a step width of 0.02° were set.
The binder screening showed that some polymers (at a printable concentration) have too low binding forces to adhere powder particles together. Examples are PCL or PEG where only damaged 3D printed probes could be retrieved from the printer. Samples printed with a sucrose based binder could be removed out of the printer without damages. With the polysaccharide binder consisting of 30 wt.% sucrose / 30 wt.% ethanol samples with the most accurate and detailed structure could be printed. In combination with HA/dextran powder it was possible to print filigree open porous scaffolds (Fig. 3). The use of isopropyl alcohol in the binder led to a swelling of the samples based on a high bleeding rate.
After sintering an obvious shrinking rate and colour shift of polysaccharide samples were detected. For the 30 wt.% sucrose / 30 wt.% ethanol samples a mean shrinking value of 11% was measured. The XRD anal-ysis showed a significant degradation of HA samples printed with an acid based binder after sintering at 1300°C/2h. A high amount of tricalcium phosphate and calcium phosphate (brushit) were analysed. By comparing pure HA with samples printed with poly-saccharide based binders no bigger effects than the usual temperature degradation could be detected after sintering at 1425°C/2h.
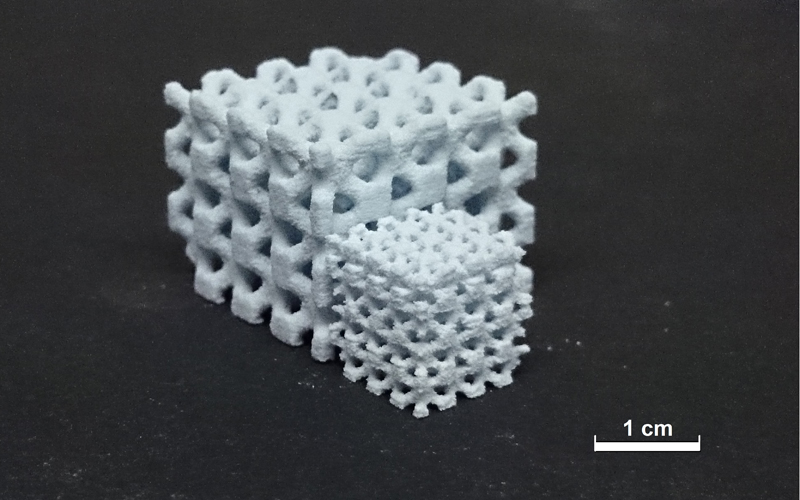
Fig. 3: Two raw 3D printed open porous HA / dextran scaf-folds.
The compressive strength (CS) of the cylinders printed with sucrose binders was too low and consequently not measureable. For cylinders printed with 35 wt.% su-crose / 32.5 wt.% ethanol the highest CS of 5.06MPa ( = 0.73) with a porosity of 66% were measured after sintering at 1425°C for 2h. Cylinders made of HA/dextran (15 wt.%) printed with the binder 25 wt.% sucrose/10 wt.% ethanol binder were found to have a high green part mean CS value (5.6MPa).
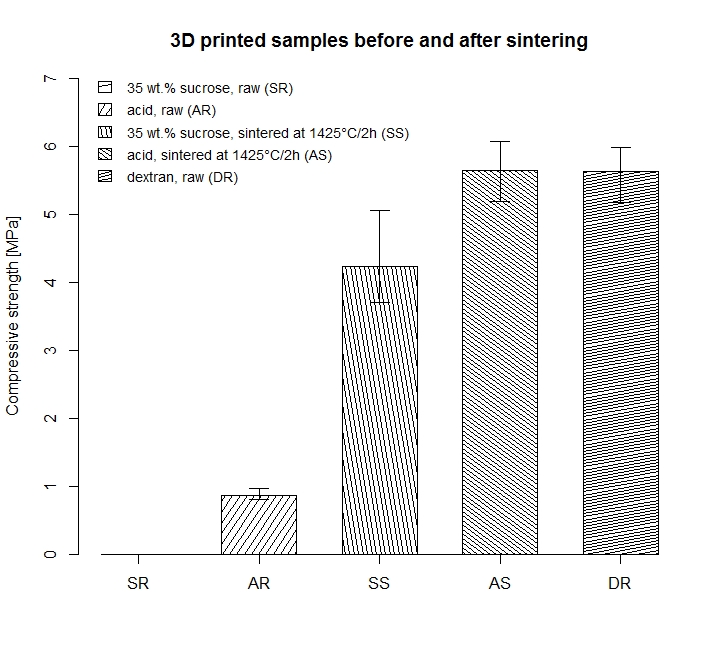
Fig. 4: The error bars indicate the highest and lowest meas-ured value.
Infiltration of the open porous scaffolds could increase the CS of an untreated sample (CS 1.0MPa, porosity: 90%) by factor 23. With the gelatine infiltration meth-od 2 (dipping) a mean CS of 23.2MPa (22% gelatine on sample) was achieved. With method 1 (after 4 runs with 12 wt.% gelatine) a mean CS of 12.6MPa was reached. Inside of the infiltrated open porous structure a homogenous infiltration took place (Fig. 1).
In the preliminary study for sintering additives an en-hanced sintering effect and an increase in CS could be shown for different mixtures after sintering at 1425°C/2h. The addition of KF (5 wt.%) to HA powder caused the biggest effect after sintering. A denser, harder ceramic with a Vickers hardness of 404HV was produced. A Vickers hardness of 357HV for 5 wt.% K2CO3 and 224HV for Na2CO3 were measured com-pared to pure HA with a value of 12HV.

Fig. 5: SEM picture of pure sintered HA (left) and HA / 5 wt.% KF sample (right) after sintering at 1425°C for 2h.
With sucrose binders, solid samples could be printed which had no defects after removing out of the printer. Different solvents showed a high bleeding during the printing process which resulted in swollen green parts and lower sintering activity. With the HA/dextran mix-ture solid green parts could be printed which had a comparable CS to sintered samples printed with an acid binder. The major advantage of polysaccharide binders is that no thermal degradation occurs during sintering in contrast to acid printed samples. Further-more, through the burn out of the polysaccharides no detectable amount of foreign material remains in the HA after sintering. With this knowledge a better con-trol and handling of HA implant manufacturing is giv-en.
With the gelatine infiltration high loadable structures were manufactured. A maximum CS of 23.2MPa was reached after vacuum infiltration with 10 wt. % gela-tine. The specimens could be infiltrated completely with both methods. The porosity of the infiltrated sam-ples was not measured, because the gelatine closed all pores hence no liquid could be used for porosity measurements. Based on these results further infiltra-tion studies will be made.
The highest sintering activity could be measured with the additives KF and K2CO3 where the Vickers hard-ness and surface structure changed most. All additives increased the hardness and the microstructure signifi-cantly compared to pure HA. It was shown that the targeted contamination influenced the sintering pro-cess. The additives lead to a liquid phase sintering where compounds with a lower melting point than HA are liquid at the sintering temperature. In a next step, analysis of chemical compositions after sintering as well as 3D printing trials with additives will be execut-ed.
Support by the Swiss-Nanoscience Institute (SNI) and Swiss National Science Foundation (SNSF) is grateful-ly acknowledged.
[1] S. Stavanovic, P. Chavanne, O. Braissant, U.Pieles, P. Gruner and R. Schumacher, 2013. Improvement of mechanical properties of 3D printed hydroxyap-atite scaffolds by polymeric infiltration, ISACB 2013
[2] E. Wintermantel and S.-W. Ha, Medizintechnik, 5. Auflage. Berlin, Heidelberg: Springer Berlin Hei-delberg, 2009.
[3] Kalita, S. J. et al., 2004. CaO–P2O5–Na2O-based sintering additives for hydroxyapatite (HAp) ce-ramics. Biomaterials, 25(12), 2331–2339. doi:10.1016/j.biomaterials.2003.09.012
[4] Suchanek, W. et al., 1997. Hydroxyapatite ceram-ics with selected sintering additives. Biomaterials, 18(13), 923–33. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9199762
Adrian Michael Rohner
University of Applied Sciences and Arts Northwestern Switzerland
School of Life Sciences
Institute for Medical and Analytical Technologies
Medical Additive Manufacturing
Gründenstrasse 40
CH-4132 Muttenz
E-Mail:
adrian.rohner@fhnw.ch
Am Projekt beteiligt
Philippe Chavanne
University of Applied Sciences and Arts Northwestern Switzerland
School of Life Sciences
Institute for Medical and Analytical Technologies
Medical Additive Manufacturing
Gründenstrasse 40
CH-4132 Muttenz
E-Mail:
philippe.chavanne@fhnw.ch
Sabrina Stevanovic
FHNW
Institute for Chemistry and Bioanalytics
Gründenstrasse 40
4132 Muttenz
E-Mail:
sabrina.stevanovic@students.fhnw.ch
Ralf Schumacher
University of Applied Sciences and Arts Northwestern Switzerland
School of Life Sciences
Institute for Medical and Analytical Technologies
Medical Additive Manufacturing
Gründenstrasse 40
4132 Muttenz
E-Mail:
ralf.schumacher@fhnw.ch
Prof.Dr. Uwe Pieles
University of applied Sciences and Arts Northwestern Switzerland
School of Life Sciences
Inst. of Chemistry and Bioanalytics -
Material-Sciences and Nanotechnology
Gründenstrasse 40
CH 4132 Muttenz
Phone: +41614674453 Mobile: +41793583380
Mail:
uwe.pieles@fhnw.ch
Industriepartner
Philipp Gruner
CEO
Medicoat AG
Almuesenacherstrasse 2a
5506 Mägenwil / Switzerland
E-Mail:
ph.gruner@medicoat.ch